Group 17 Elements :
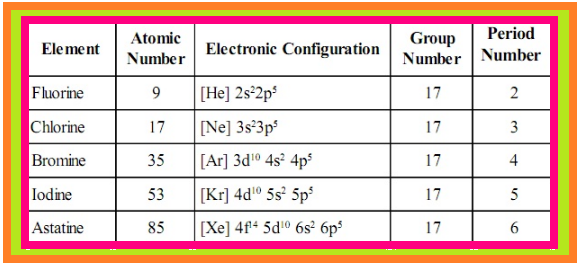
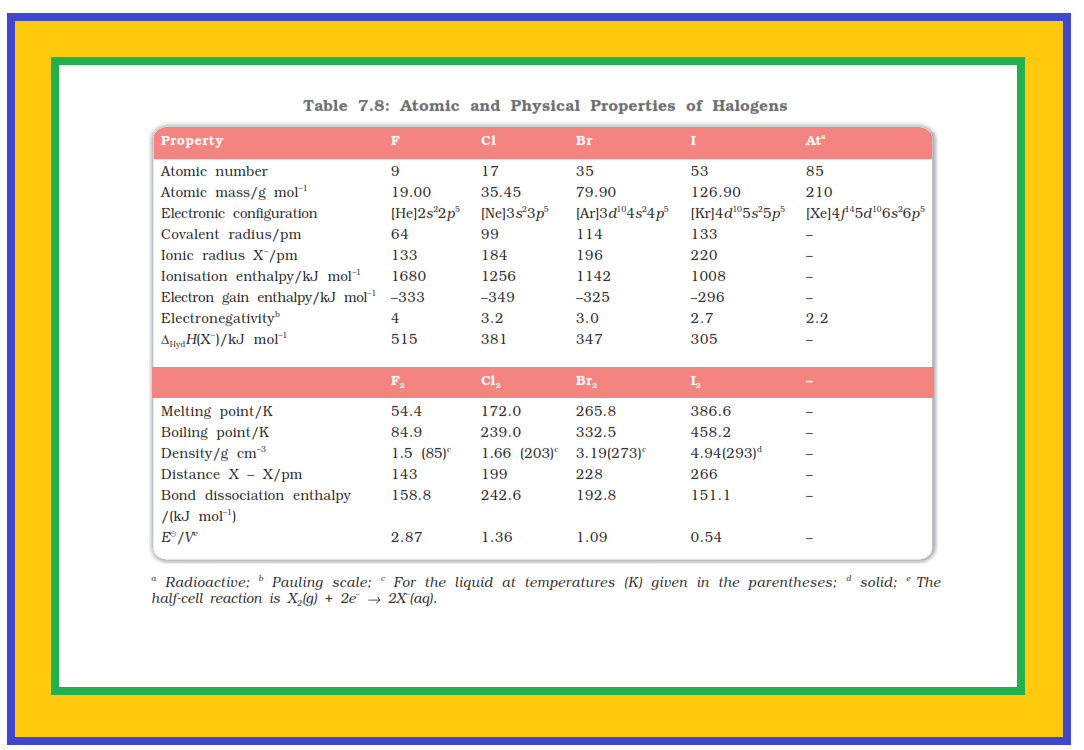
`=>` Fluorine, chlorine, bromine, iodine and astatine are members of Group 17.
`=>` These are collectively known as the halogens (Greek halo means salt and genes means born i.e., salt producers).
`=>` The halogens are highly reactive non-metallic elements.
`=>` Like Groups 1 and 2, the elements of Group 17 show great similarity amongst themselves. That much similarity is not found in the elements of other groups of the periodic table.
`=>` There is a regular gradation in their physical and chemical properties.
`=>` Astatine is a radioactive element.
`=>` These are collectively known as the halogens (Greek halo means salt and genes means born i.e., salt producers).
`=>` The halogens are highly reactive non-metallic elements.
`=>` Like Groups 1 and 2, the elements of Group 17 show great similarity amongst themselves. That much similarity is not found in the elements of other groups of the periodic table.
`=>` There is a regular gradation in their physical and chemical properties.
`=>` Astatine is a radioactive element.